Abstract
Introduction: Progressive disease post autologous CD19 CAR T treatment, due to antigen escape, is a reality that demands the implementation of rapidly accessible mitigation strategies. Allogeneic CAR T cells produced by healthy donors potentially offer a rapid alternative to autologous strategies; and the clinical success of autologous CD22 CAR T therapeutics provides a route to supplement CD19 CAR treatment and potentially overcome CD19 antigen escape. Here we describe methods to create a hypoimmune, allogeneic CAR T cell product to mitigate GvHD via TRAC gene disruption, support adaptive immune cell evasion via B2M and CIITA gene disruption, and promote persistence and innate immune cell evasion by overexpression of CD47. Tethered with dual-transduction to create CD19 CAR x CD22 CAR T cells overexpressing CD47, our described hypoimmune, allogeneic CAR T cell approach aims to overcome antigen escape and create CAR products with enhanced clinical durability.
Methods: Healthy donor T cells, obtained by leukapheresis, were engineered to evade innate immunity by transduction with lentivirus encoding CD47 appended to a second-generation CD19 CAR or CD22 CAR to create dual-transduced CD19 CAR x CD22 CAR T cells overexpressing CD47. Control CAR T cells (single CD19 CAR T or CD22 CAR T overexpressing CD47) were similarly produced and subjected to functional in vitro and in vivo analyses against NALM and RAJI parental (CD19+/CD22+) or antigen knockout (CD19- or CD22-) leukemia and lymphoma cells, respectively. Efficiency of transduction was analyzed by flow cytometry and VCN, while cytotoxicity and cytokine production were performed by IncuCyte and meso scale discovery analysis, respectively. Systemic NALM and RAJI parental or antigen knockout tumor models (IV injection) were executed in a NSG mouse background.
Single CAR control and dual-transduced CD19 CAR x CD22 CAR T cells overexpressing CD47 were generated as above and further engineered using electroporation and CRISPR-Cas12b to disrupt the B2M, CIITA, and TRAC (TCR) genes to generate hypoimmune versions of these CAR T cells. TCR depleted CAR T cells were subjected to in vitro and in vivo analyses, as above, using non-hypoimmune CAR T cells as controls.
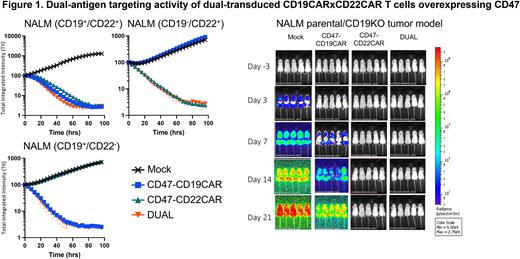
Results: To optimize the percentage of dual-transduced CD19 CAR x CD22 CAR T cells, we performed a lentivirus titration matrix on Pan T cells. At our described dose, we created CAR T cells (n=3) that were 45.9 ± 7.2% CD19 CAR x CD22 CAR and CD47-overexpressing. Dual-transduced and sorted CD19 CAR x CD22 CAR T cells overexpressing CD47 reduced tumor counts at E:T ratios at and below 0.625:1 and higher cytokine (IFNy | GM-CSF | IL2 | TNFa) at E:T ratios as low as 0.08:1 relative to single CAR controls. NALM and RAJI CD19 knockout in vivo tumor models demonstrated that dual-transduced CAR T cells significantly reduced flux (Day 21 AUC vs Mock: NALM (p=0.0001) | RAJI (p=0.0001)) and prolonged survival (Median vs Mock: p<0.002 | vs CD19CAR: p<0.015). Hypoimmune CD19 CAR x CD22 CAR T cells similarly elicited cytotoxicity and cytokine responses in vitro comparable with non-hypoimmune CD19 CAR x CD22 CAR T cells.
Summary: Dual-transduced CD19 CAR x CD22 CAR T cells efficiently control NALM and RAJI antigen knockout models in vitro and in vivo. Studies that separated dual-transduced CAR T cells into single (CD19 CAR T or CD22 CAR T) or dual CD19 CAR x CD22 CAR T expressing populations indicated that CD19 CAR x CD22 CAR T cells better control tumor growth and elicit higher levels of cytokine response than single CAR controls at low E:T ratios in vitro. Furthermore, dual-transduced, or dual-transduced and sorted, CD19 CAR x CD22 CAR T cells overcome antigen escape and better control NALM tumor growth in vivo relative to a combined product of single CD19 CAR and CD22 CAR T cells. Studies that evaluate evasion of our hypoimmune CD19 CAR x CD22 CAR T cells overexpressing CD47 from adaptive and innate immune cell recognition are ongoing. Our group has previously described how hypoimmune CD19 CAR T cells are functionally immune evasive and may be an attractive alternative to current autologous or allogeneic CD19 CAR T cell approaches. Likewise, these described CD19 CAR x CD22 CAR T cell studies may provide universal CAR T cells that persist without immunosuppression and simultaneously mitigate potential CD19 antigen escape.
Disclosures
Johnson:Sana Biotechnology, Inc: Current Employment, Current equity holder in publicly-traded company. Wright:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Hu:Sana Biotechnology Inc: Current Employment, Current equity holder in publicly-traded company. van Hoeven, PhD:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Granger:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Liang:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Moreno:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Lamba:Sana Biotechnology, Inc: Current Employment, Current equity holder in publicly-traded company. Young:Sana Biotechnology Inc: Current Employment, Current equity holder in publicly-traded company. McAlister:Sana Biotechnology, Inc.: Current Employment, Current equity holder in publicly-traded company. Fry:Sana Biotechnology: Current Employment, Current equity holder in publicly-traded company. Schrepfer:Sana Biotechnology Inc: Current Employment, Current equity holder in publicly-traded company. Foster:Sana Biotechnology Inc: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal